Amine Protection and Deprotection Master Organic Chemistry
Deprotection of OBoc and other protecting groups Download Table

I am trying to cleave a tert-butyloxycarbonyl (Boc) protecting group from an aminooxy group ($\\ce{-O-NH-Boc}$) with trifluoroacetic acid (TFA) in $\\ce{CH2Cl2}$ but i get minimum product and too much
Amine Protection and Deprotection Master Organic Chemistry
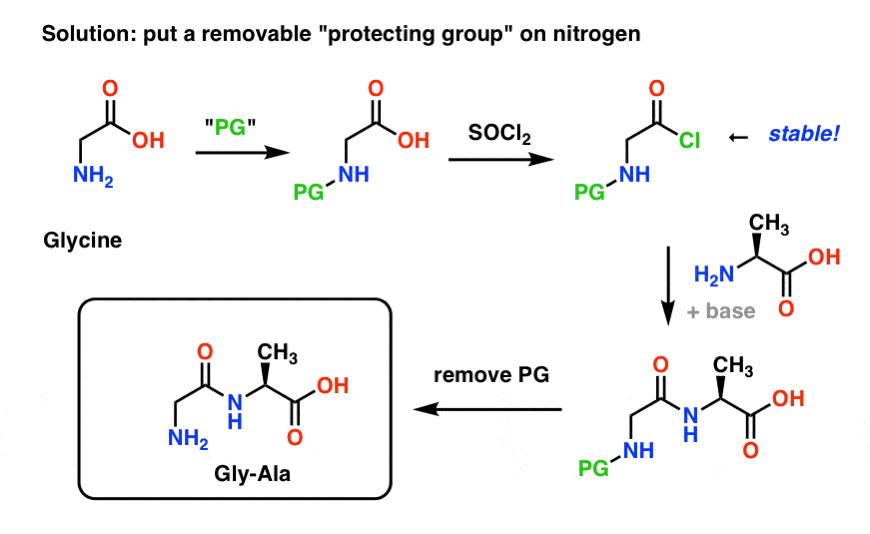
Steps: The amine attacks a carbonyl site on di-tert-butyl dicarbonate resulting in tert-butyl carbonate leaving as a leaving group.tert-Butyl carbonate picks up the proton from the protonated amine.; tert-Butyl bicarbonate breaks down into CO2 (gas) and tert-butanol.; Key Points: The CO2 gas that forms during the reaction should be allowed to escape. Don't run boc protections in closed system
Protecting Groups for Amines Carbamates Master Organic Chemistry
Boc-Protected Amino-Groups in Multi-step Syntheses. SmCl 3 is an excellent catalyst for chemoselective esterifications and selective removal of acid sensitive hydroxyl protecting groups such as Boc, THP, and TBDMS. Chemoselective deprotection is demonstrated through suitable examples.
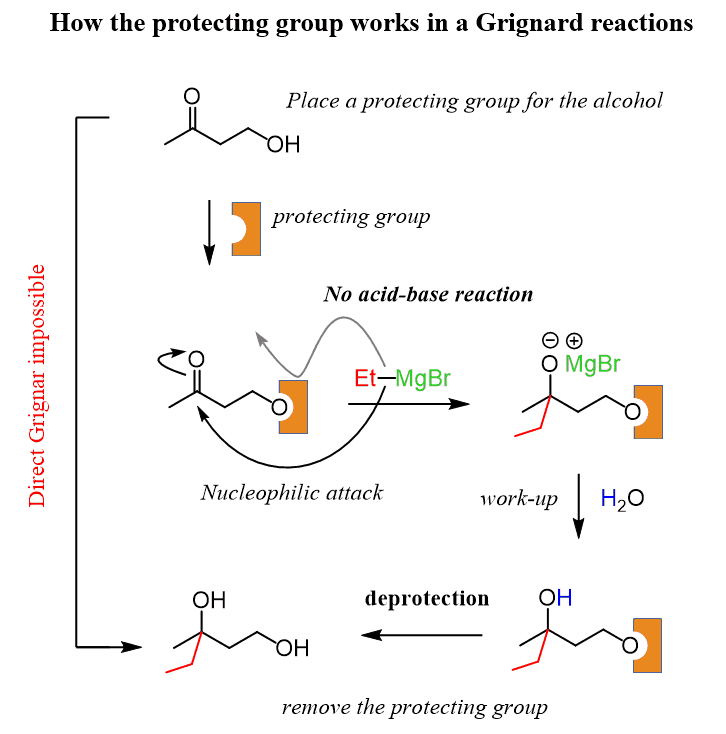
Protecting Groups For Alcohols Chemistry Steps
tert-Butyloxycarbonyl protecting group. The tert-butyloxycarbonyl protecting group or tert-butoxycarbonyl protecting group (BOC group) is a protecting group used in organic synthesis.. The BOC group can be added to amines under aqueous conditions using di-tert-butyl dicarbonate in the presence of a base such as sodium hydroxide: . Protection of amines can also be accomplished in acetonitrile.
On the Selective NMethylation of BOCProtected Amino Acids The Journal of Organic Chemistry

(Boc) group is one of the classical masking functionalities employed in organic synthesis for the protection of amino groups.3-5 Boc ful lls this requirement of a 'good' protecting group, and is preferred in amino protection because of its stability to nucleophilic reagents, hydrogenolysis and base
Protecting Groups for Amines Carbamates Master Organic Chemistry
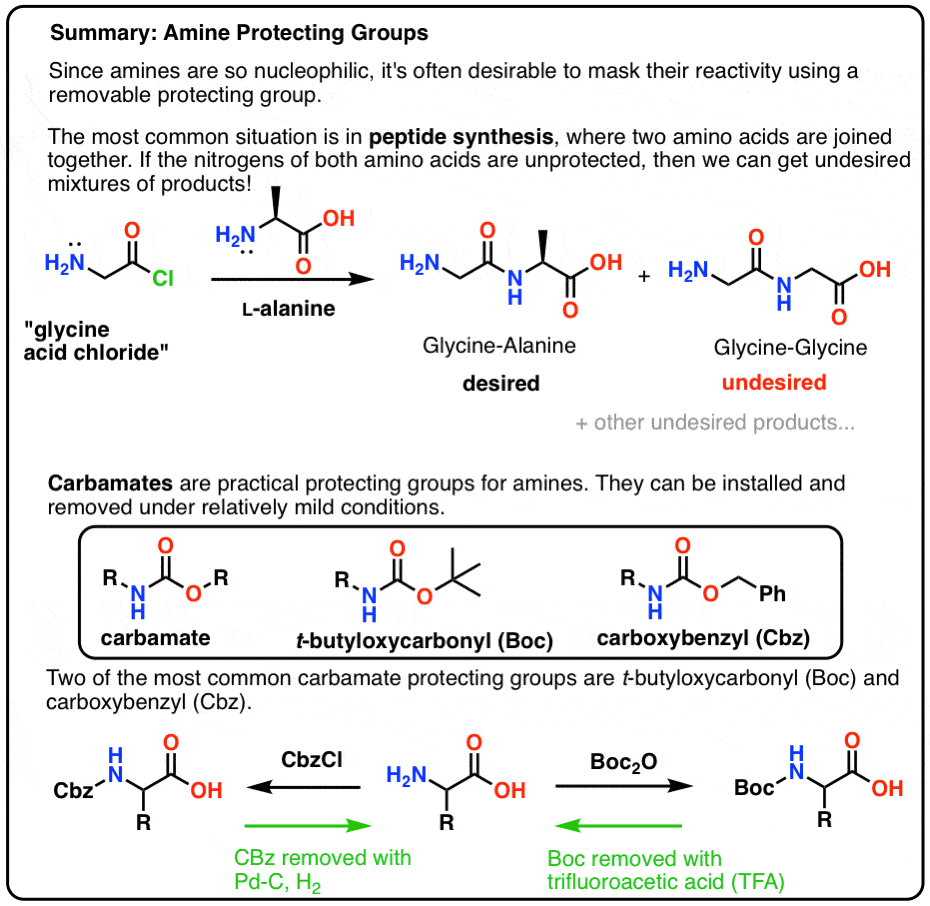
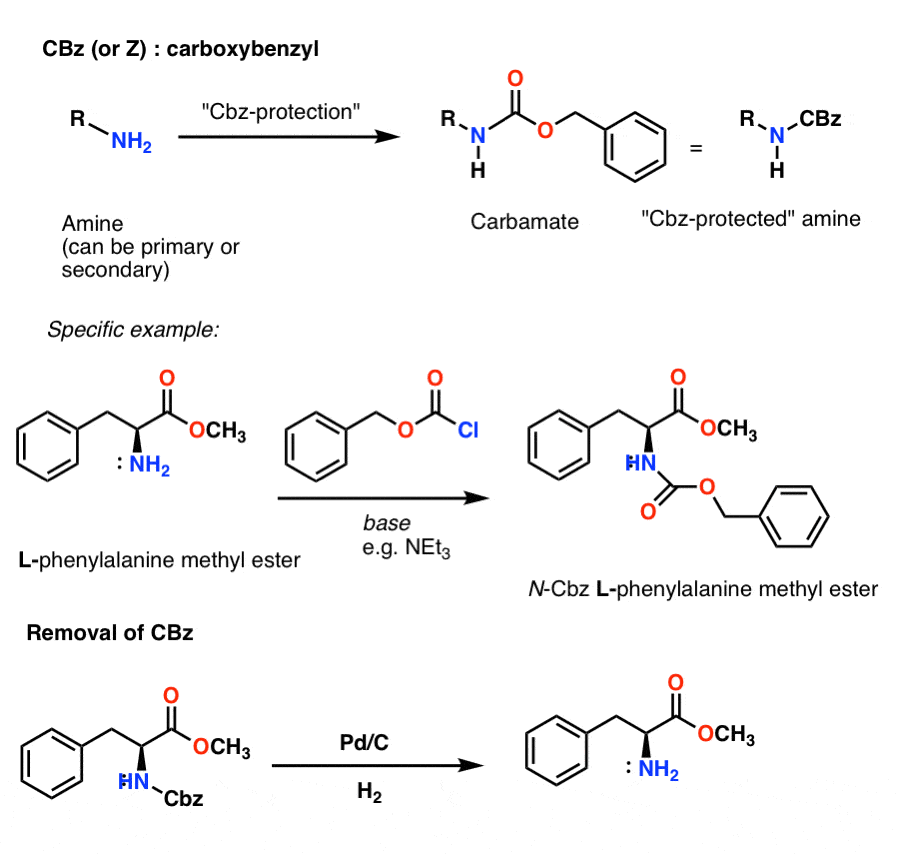
The Boc group is the most used protection of amino groups for example in the synthesis of peptides, but let's also discuss the phenylmethoxycarbonyl group (abbreviated carbobenzoxy or Cbz). The benzyl group is usually stable under acidic and basic conditions and is cleaved by catalytic hydrogenation with H 2 over Pd/C.
Protecting Groups for Amines Carbamates Master Organic Chemistry
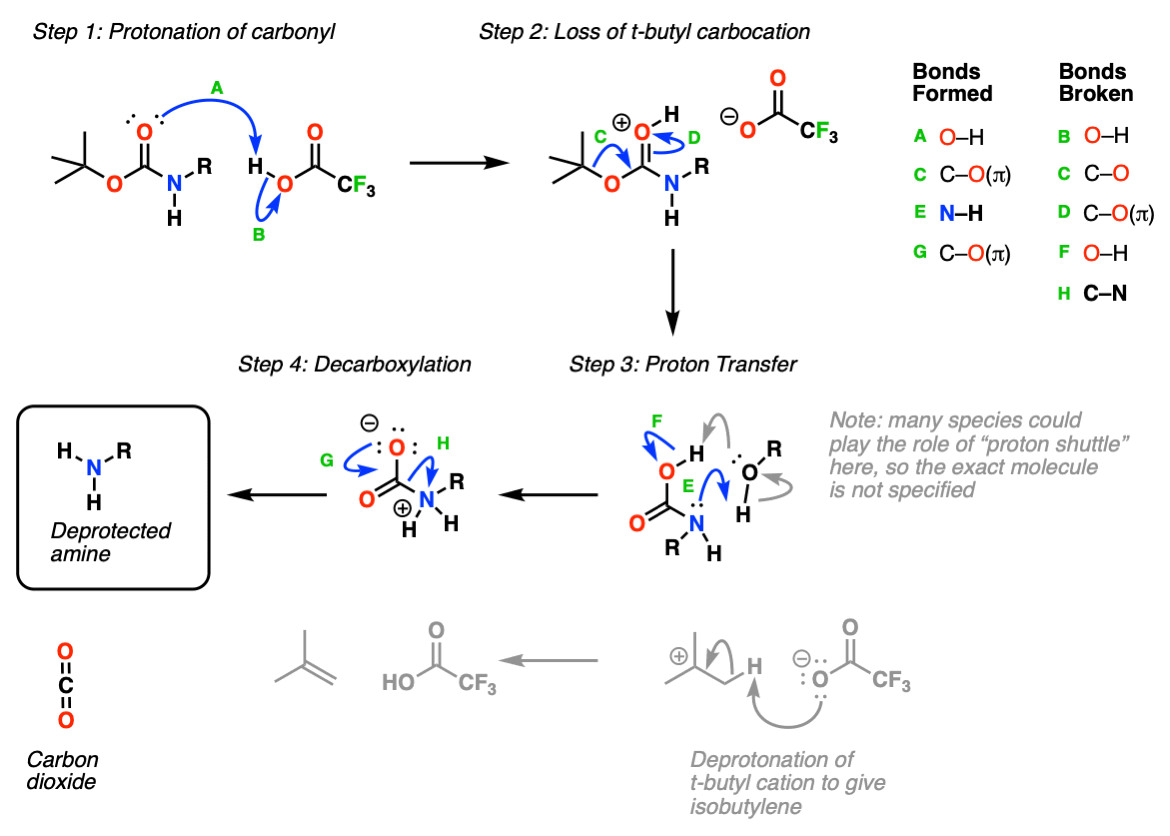
Mechanism: Steps: The tert -butyl carbamate becomes protonated. Loss of the tert -butyl cation results in a carbamic acid. Decarboxylation of the carbamic acid results in the free amine. Protonation of amine under the acidic conditions provides the pdt as the TFA salt. Key Points: The tert -butyl cation will either be quenched by a suitable.
Boc Deprotection Mechanism Organic Chemistry YouTube

Installation and Removal of the "Boc" Protecting Group The Boc group is usually installed with "Boc 2 O" (sometimes referred to as "Boc anhydride"), and is removed with acid. The usual choice is "neat" (i.e. undiluted) trifluoroacetic acid (TFA), which pops the Boc groups off very cleanly, liberating CO 2 and t -butyl alcohol.
Boc Groups as Protectors and Directors for IrCatalyzed C−H Borylation of Heterocycles The

Other protecting group: Boc Amine PGs Introduction Cbz 2 O, Cbz‐Cl Alloc 2 O, Alloc‐Cl ivDde‐OH Removal H 2 Pd(PPh 3), PhSiH 3 2% N 2 H 4 Stable Basic and Acidic conditions Basic and Acidic conditions Basic and Acidic conditions, Hydrogenation Orthogonal Boc, Fmoc, Trt Boc, Fmoc, Trt Boc, Fmoc, Z, Trt, Alloc 4
Tale of Two Protecting Groups—Boc vs SEM—for Directed Lithiation and CC Bond Formation on a

Abstract: Fast, efficient and selective deprotection of the tert-butoxycarbonyl (Boc) group of various amino acids and peptides was achieved by using hydrogen chloride (4 m) in anhydrous dioxane solution for 30 min at room temperature. In the cases studied in our laboratory, this protocol provided superior selectivity to deprotect Nalpha-Boc groups in the presence of tert-butyl esters and tert.
Deprotection of OBoc and other protecting groups Download Table

Hydroxyl (OH) ( OH) protecting groups in Organic Synthesis. Protection of alcohols: Acetyl (Ac) ( Ac) - Removed by acid or base. Benzoyl (Bz) ( Bz) - Removed by acid or base, more stable than Ac Ac group. Benzyl ( Bn Bn, Bnl Bnl) - Removed by hydrogenolysis. Bn Bn group is widely used in sugar and nucleoside chemistry.
Protecting Groups for Amines Carbamates Master Organic Chemistry

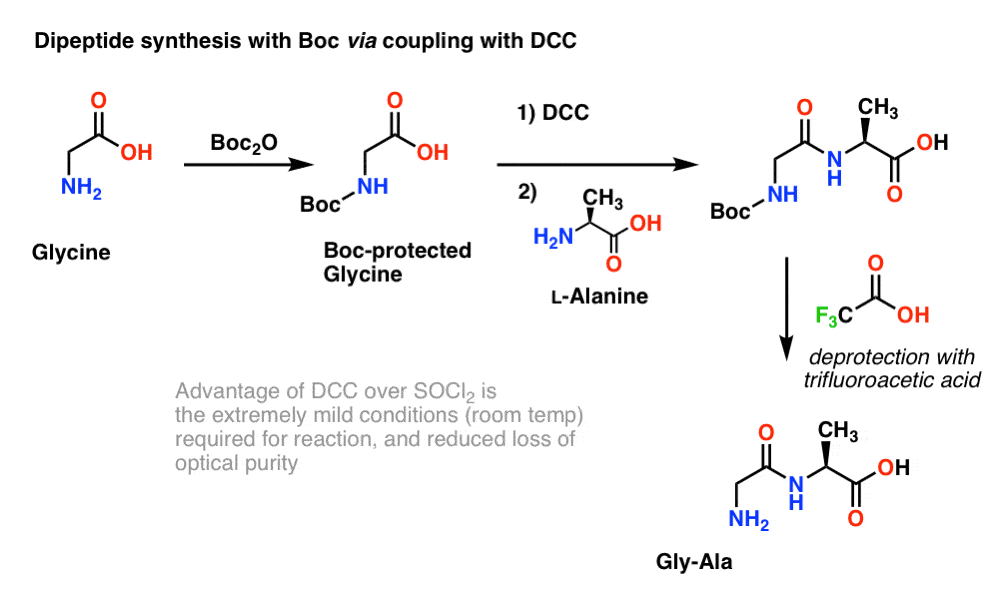
Example 5 shows removal of the CBz protecting group from the amine using catalytic hydrogenation. Mechanism: Protection of amine using Boc 2 O (Di-t-butyl dicarbonate). In the first step, the amine attacks the carbonyl carbon of the carbonate (Step 1, arrows A and B) which forms a new tetrahedral intermediate.
Carpino's protecting groups, beyond the Boc and the Fmoc El‐Faham 2020 Peptide Science

N-Acylmethionine as Amine Protecting Group The analytically important cleavage of methionine-containing peptides by cyanogen bromide (Gross and Witkop, 1962) has been applied to eliminate an A^AT-carbonylbis (methionyl) cross-linking residue from insulin (Geiger and Obermeier, 1974/77; Busse et , 1974).
Protecting Groups for Amines Carbamates Master Organic Chemistry
Amines can be protected by Boc-, Fmoc-, Cbz-, or Alloc protecting groups, which are removed by acids, bases, hydrogenolysis, or the use of transition metals respectively.. The removal of the amine protecting Fmoc is initiated by abstraction of a relatively acidic C-H proton, immediately followed by elimination (E1cB mechanism). The.
BOC Protection and Deprotection J&K Scientific LLC

A removal of Z group stimulated further research toward acid-sensitive protecting groups. This led to development of series of blocking groups cleavable under mild conditions. The most important were for instance, tert-butyloxycarbonyl (Boc), O-nitrophenylsulfenyl (Nps) and biphenylylisopropyloxycarbonyl (Bpoc) groups.
What is solid phase peptide synthesis?

A highly selective and efficient deprotection of the N‐t‐butoxy carbonyl (N‐Boc) group on indoles, pyrroles, indazoles, and carbolines has been achieved in high yields using a catalytic amount of NaOMe as a base in dry MeOH, at ambient temperature. IICT Communication No. 060512.